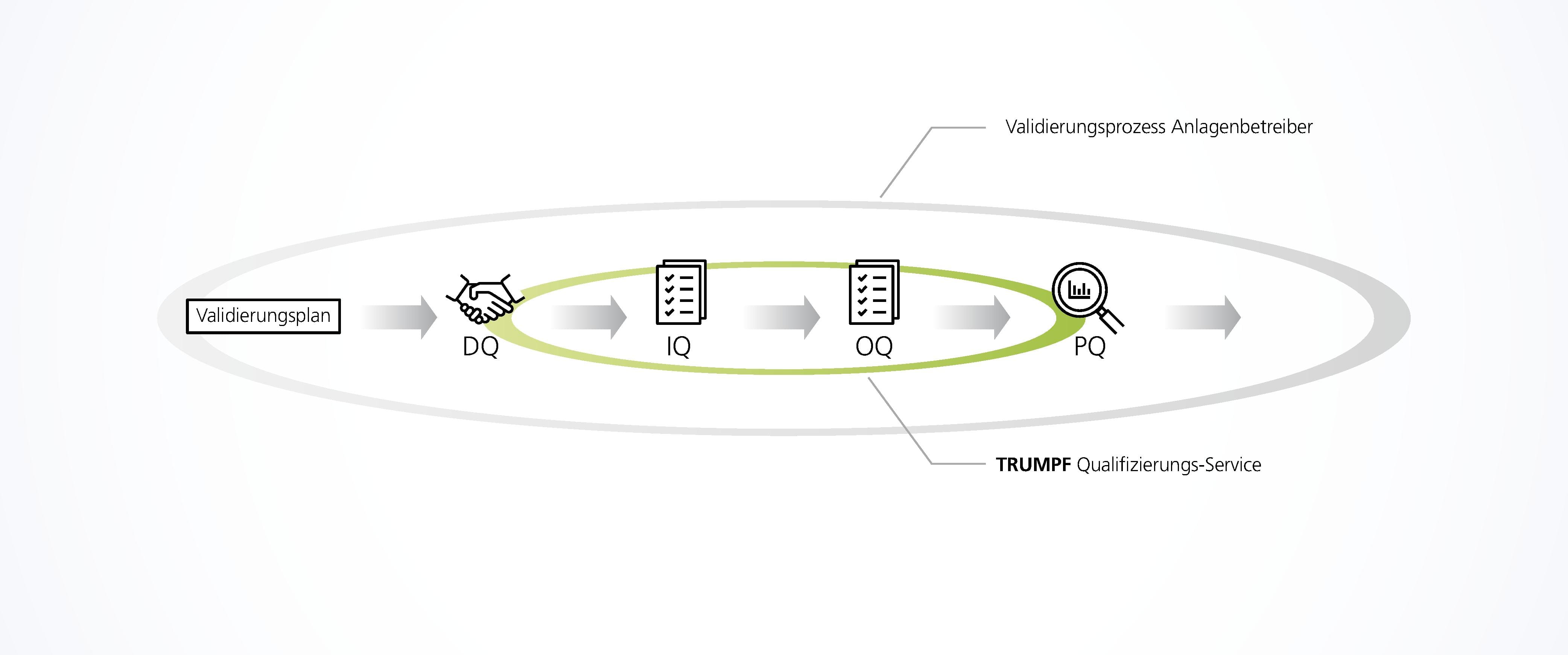
Machine qualification: IQ and OQ
Equipment such as TRUMPF marking lasers must also be qualified as part of the process validation in order to comply with the legal MDR regulation (Medical Device Regulation) or the Medical Device Directive 93/42/EEC.
TRUMPF supports you as a reliable partner: installation and functional qualification on site. TRUMPF experts carry out the qualification step-by-step directly on the system using comprehensive qualification documentation.
Installation Qualification (IQ): Inspection of the correct delivery (conformity with offer), installation, documentation of the system as well as the measuring equipment used.
Operational Qualification (OQ): Inspection of the correct basic functions of the system in the selected work environment. This will verify that the entire laser marking system functions according to the defined specifications.