Determination of particle cleanliness of a medical device
Particulate impurities in the medical device used endanger the safety of the patient. A tailor-made method was developed for the manufacturer of an abdominal dressing set to identify the particulate impurities and evaluate them toxicologically.
Manufacturers of medical devices are responsible for ensuring that their products do not jeopardize the safety of patients or third parties when used as intended. Our customer is a manufacturer of an abdominal dressing kit. This dressing kit contains a polymer film which, when applied, touches the organs in the abdominal cavity. The cleanliness of this polymer film is thus essential. In order to obtain FDA approval for its abdominal dressing kit, the manufacturer had to prove that the amount and type of visible and sub-visible particulate contaminants on the film does not pose any health risk for the patients.
To date, there is no standard that specifies exactly how particulate contaminants on medical devices should be identified. We have therefore developed a tailor-made method in close cooperation with the manufacturer of the abdominal dressing kit.
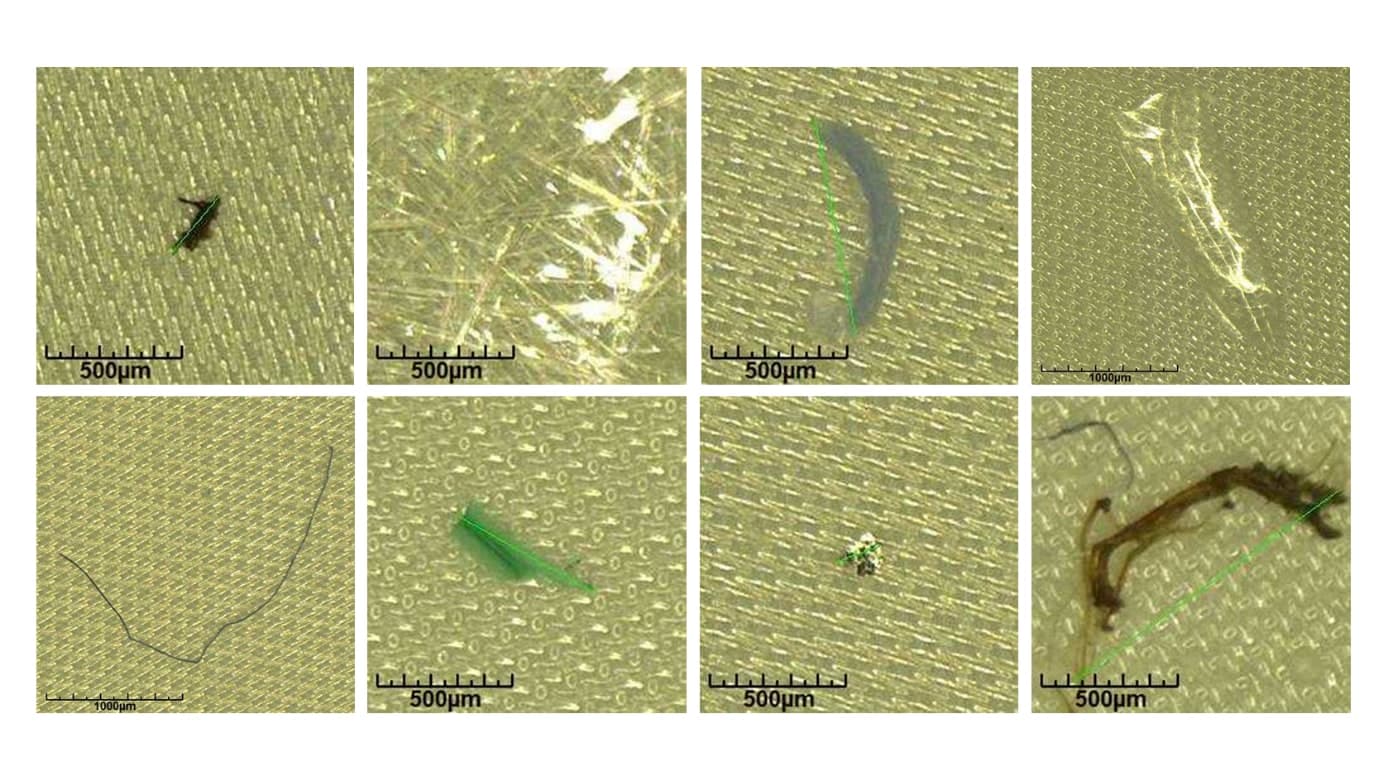
Basically, the process can be divided into the following steps: Extraction of the medical device, filtration of the extract, counting and sizing of the particles, and chemical characterization of the particles.
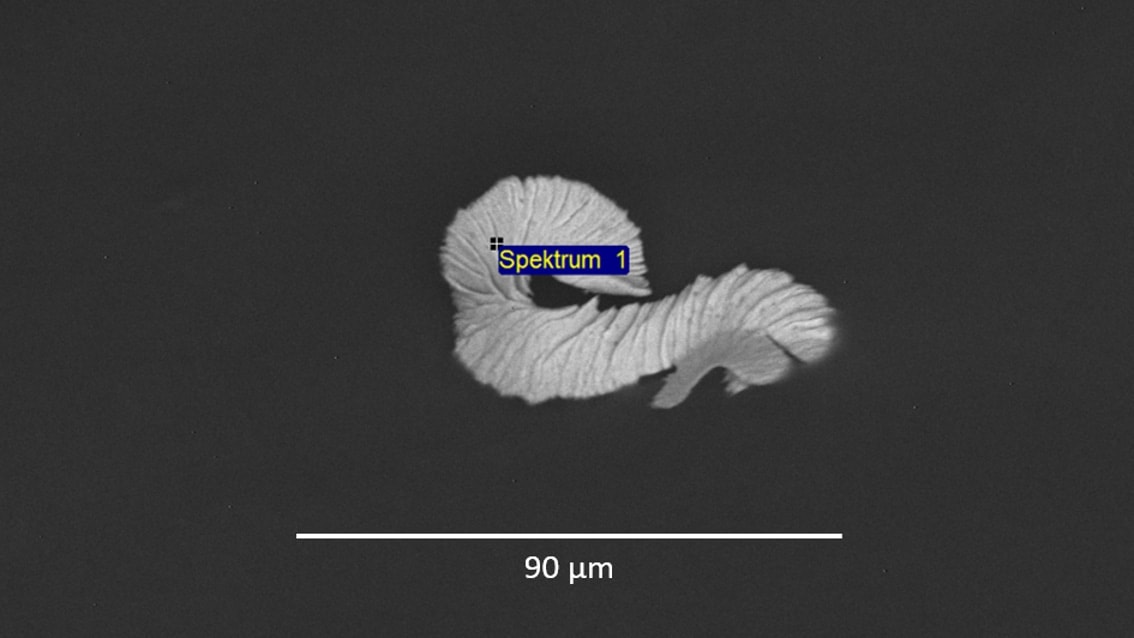
As required by the FDA, we have performed the extraction according to ISO 10993-12:2012 "Biological evaluation of medical devices - Sample preparation and reference materials". For counting, sizing and grouping (metallic particles, non-metallic particles or fibres) of the particles, we used an automated analysis system based on light microscopy. The use of such systems is recommended in VDA 19.1 "Quality Management in the Automotive Industry - Inspection of Technical Cleanliness ", has proven itself there and corresponds to the state of the art. The chemical characterization of the particles was done using FTIR and EDX according to ISO 10993-18:2005 "Biological evaluation of medical devices - Chemical characterization of medical device materials within a risk management process".
All particles extracted from the polymer film could be assigned either to the polymer film itself, to the other components of the dressing kit, to the production environment, to the packaging, or to the personal protective equipment. The subsequent toxicological evaluation of the amount and type of particulate contaminants performed according to ISO 10993-17:2002 "Biological evaluation of medical devices - Establishment of allowable limits for leachable substances" revealed that the health risks were negligible.
The results generated in this study completed the cleanliness report of the finished medical device. Soon after, the manufacturer received FDA approval.