Injection Moulding Engineered for Precision
Micron-level accuracy, ISO 13485-certified compliance, and process stability across the full product lifecycle. Our injection moulding solutions are engineered to meet the highest regulatory, functional, and cleanliness requirements of the medical industry.
Cleanroom ISO-7 Injection Moulding
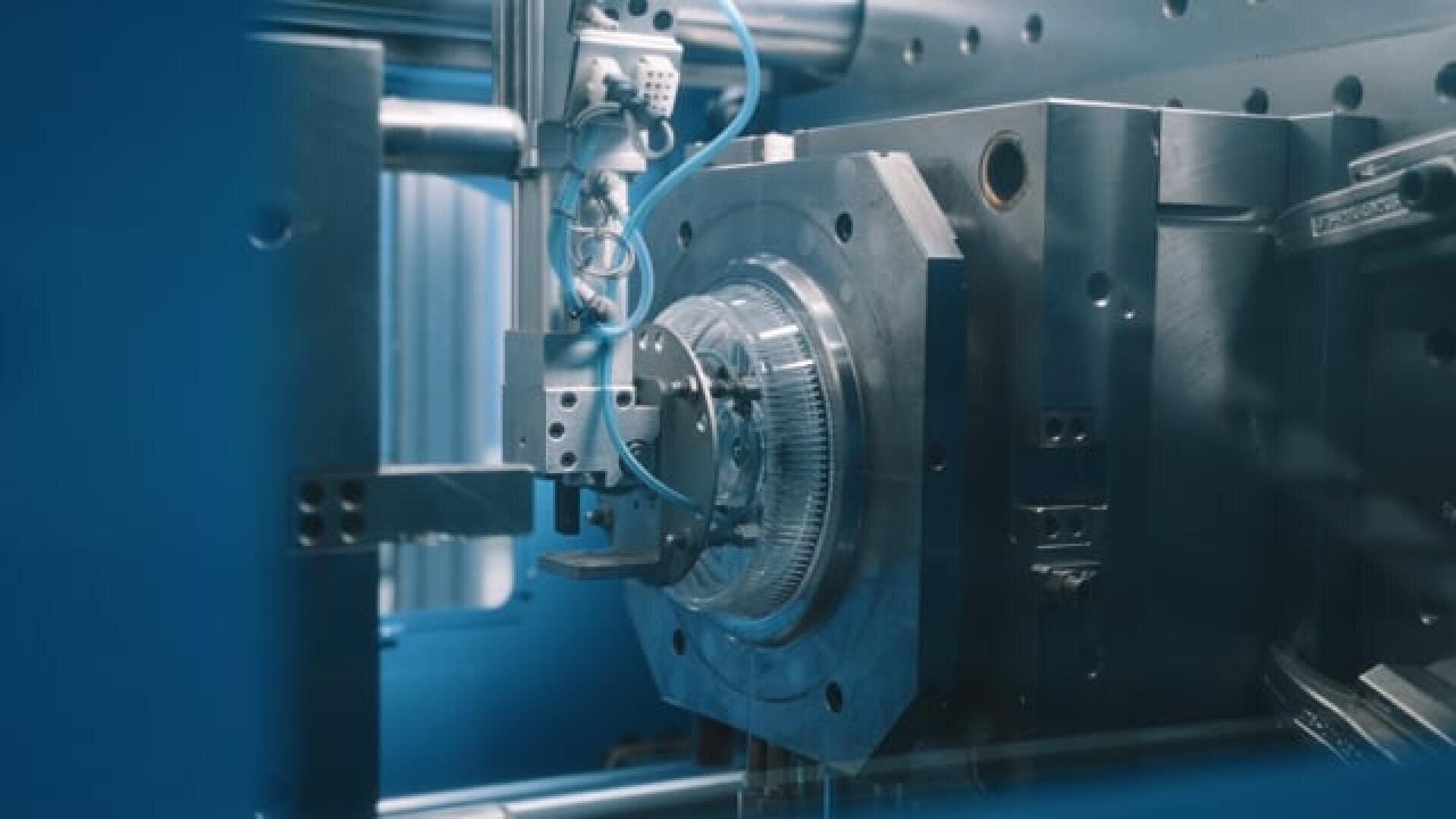
Our ISO 7 cleanroom integrates high-performance injection moulding cells engineered for validated medtech production. The environment is fully monitored for air purity, microbial load, and particulate control, ensuring ISO 13485 compliance, traceability, and cleanroom transfer readiness from first shot to scale-up.
- ISO-7 Cleanroom Injection Moulding - We manufacture Class I and II medical components in ISO Class 7 cleanroom environments, following validated IQ/OQ/PQ protocols and ensuring fully traceable documentation. All tooling is qualified for cleanroom transfer, material compatibility, and sterility assurance at every stage.
- Automated Process - We deploy fully automated moulding cells combining robotics, servo-electric injection, cavity pressure sensors, and closed-loop process control. Designed for medtech scale-up, this system ensures statistical repeatability and fast cycles.
Process & Product Validation
Our injection moulding processes are built for full regulatory traceability and aligned with Good Manufacturing Practices (GMP). From design qualification to scale-up, each product undergoes a rigorous validation path.
- Design Qualification
- Factory Acceptance Test
- Mould Validation Validation Protocol
- Instalation Qualification
- Process Development with Design Experiments
- Operational Qualification
- Process Qualification
- Start of Production with validated protocols
Advanced Injection Moulding for Functional Integration
We integrate functionality during moulding to eliminate secondary operations and streamline compliance. From overmoulding and in-mould labelling to embedded sensors, each process is controlled by scientific moulding protocols and executed in ISO 7 environments. The result: validated, traceable, and cleanroom-ready components, produced with repeatability and regulatory alignment.
- Scientific Moulding Approach
- White Room for Assembly
- Functional Moulding Integration