Powerful and regulatory-compliant medical device software (MDSW) from a single source
Medical device software (MDSW) must not only be technically convincing but also regulatory compliant – this requires experience, expertise, and a well-coordinated team. This is precisely where our partnership between ISS AG and ERNI Schweiz AG comes into play.
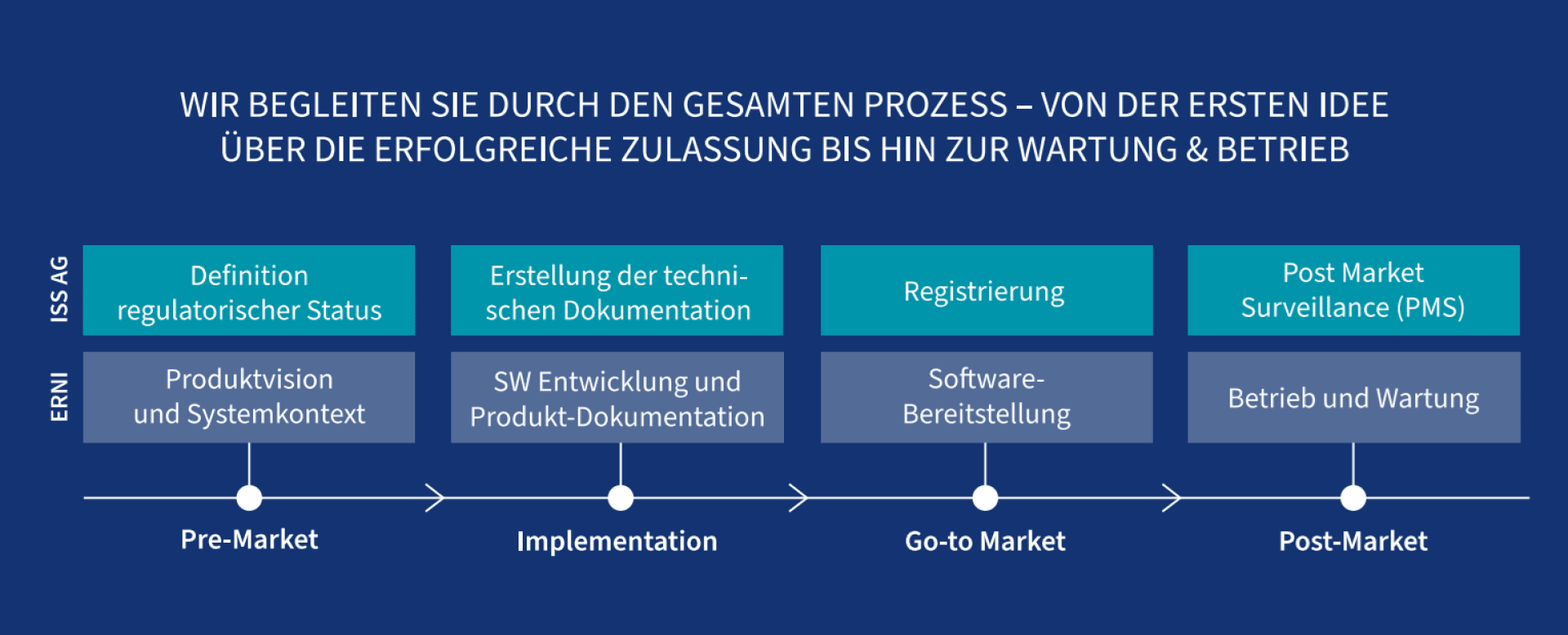
Pre-Market
We define the intended use of your software with you and analyze the target market as well as the system landscape. From this, we derive regulatory requirements and lay the foundation for successful approval—both nationally and internationally.
Together with you, we determine the purpose of your software, including target audiences, areas of application, and desired outcomes, and analyze the target market. We clarify whether your software is a medical device or an IVD (In Vitro Diagnostic) and into which risk class it falls (classification). Additionally, we define your role as an economic operator and the resulting obligations.
Based on this, we develop a clear regulatory strategy, formulate the product vision, and take into account technical, user-related, and regulatory frameworks. This lays the foundation for successful approval—both nationally and internationally.
Implementation
While we ensure agile and technically modern software development based on intended use and regulatory requirements, ISS AG supports the creation of the necessary technical documentation.
This includes risk assessments, requirements, testing, and traceability, all in accordance with MDR, IVDR, and other relevant regulations.
Furthermore, ISS AG ensures software and cybersecurity compliance and develops the clinical evaluation strategy. Together with you, we define a market launch strategy that takes into account all roles, markets, and regulatory pathways.
Go-to-Market
Now it gets serious: ISS AG coordinates the registration of your medical device software (MDSW), ensures compliance with all documentation requirements, and guides you through the approval process. At the same time, ERNI delivers a ready-to-use, tested software solution including user documentation.
Medical devices, including software, must be registered and approved before market launch, depending on the country. ISS AG ensures that all requirements are met, conducts clinical studies if necessary, and submits the required documents for registration. ERNI takes care of the operational part: The software is provided in the cloud, regularly updated, and technically supported.
Post-Market
This ensures that your product remains safe, compliant, and future-proof.
Even after the market launch, we remain by your side: ISS AG takes care of regulatory updates, product changes, manages PMS strategies, conducts vigilance reporting, and provides support during audits, complaints, and cybersecurity issues.
Why choose ERNI & ISS?
Your path to market success – step by step.
Your advantages at a glance
|
|
Ready for the next step?
Let us realize your MDSW vision together.
Whether a startup or an established company – we develop safe, powerful, and compliant solutions for the healthcare market of tomorrow.